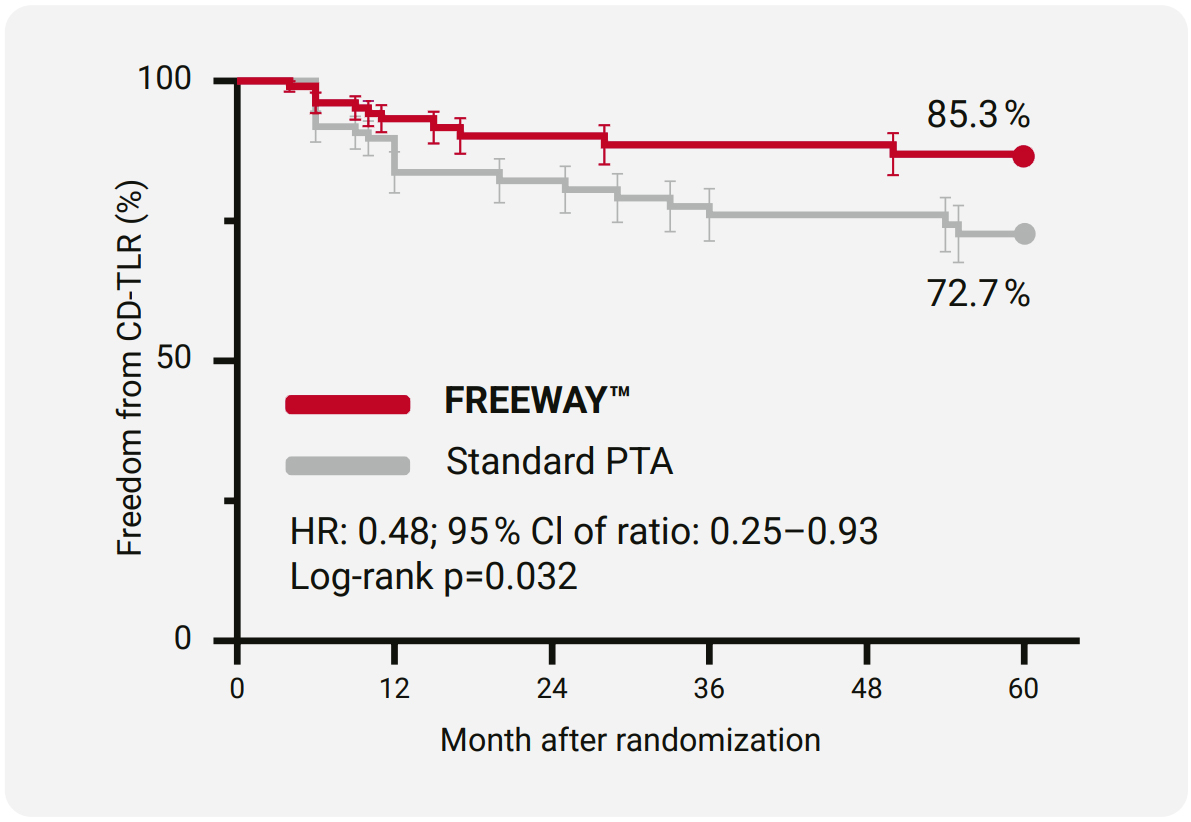
5 Year Freedom from CD-TLR
Study design & focus
- 13 centers in Austria and Germany
- 148 patients with de novo or restenotic lesions that needed stent implantation
- long term follow-up, 5 years and beyond, of the randomized Freeway Stent Study²
FREEWAY™ – CLINICAL PROGRAM